WHAT IS A CATALYTIC CONVERTER? WHAT DOES IT DO? HOW DOES IT WORK?

Cars generate a great deal of fumes and gases, also known as emissions, when they’re running. Emissions contribute to air pollution, which is why the Clean Air Act was passed in the United States in 1963 to help reduce the amount of pollution produced by a range of industries.
The National Emissions Standards Act, an amendment made in 1965 to the Clean Air Act, set the first federal vehicle emissions standards. Each state has regulations that adhere to federal standards, with many states requiring that all registered cars be tested to evaluate the output of emissions.
The modern exhaust system on vehicles features a range of parts that helps control emissions and make them more environmentally friendly. Among these parts is a catalytic converter, which helped many car manufacturers meet the standards set by the National Emissions Standards Act.
A catalytic converter is an important part of the exhaust system and by now you're probably wondering what does a catalytic converter do? Keep reading to find out more about the role it plays in your car.
What Is a Catalytic Converter?
As mentioned above, a catalytic converter is an essential part of a vehicle's exhaust system. It helps lower the number of toxic pollutants emitted into the air by converting hazardous combustion gases into less harmful substances, like water vapor and carbon dioxide.
This is done by exposing these fumes to chemicals and metals inside the converter to prompt chemical reactions that transform otherwise toxic pollutants into relatively harmless ones. Let’s break down how a catalytic converter functions a bit further.
What Does the Catalytic Converter Do?
A catalytic converter uses a chamber called a catalyst to change the harmful compounds from an engine’s emissions into safe gases, like steam. It works to split up the unsafe molecules in the gases that a car produces before they get released into the air.
The catalytic converter is located on the underside of a vehicle and looks like a large metal box. There are two pipes coming out of it. The convertor utilizes these two pipes and the catalyst during the process of making the gases safe to be expelled.
Gases are brought in from the “input” pipe connected to the engine of a vehicle. These are blown over the catalyst, which causes a chemical reaction that breaks apart the pollutants. The less-harmful gases now travel through the second pipe, or the “output,” that is connected to a car’s tailpipe.

What Is Inside a Catalytic Converter?
So what is a catalytic converter made of? The catalyst inside a catalytic converter is made typically from platinum or a similar metal, such as rhodium or palladium. Gases flow through a ceramic honeycomb structure located within the cat housing. This is lined with metals that have specific jobs that play a role in reducing emissions. There are two main types of catalysts that might be featured in a car:
- Reduction catalysts: Help reduce nitrogen oxide pollution by removing oxygen. Nitrogen oxides are broken up into nitrogen and oxygen gases, which on their own are harmless.
- Oxidation catalysts: Used to change carbon monoxide into carbon dioxide through an opposite process of adding oxygen.
Also located near the catalytic converter is an oxygen (O2) sensor, which works to tell a car’s electronic control unit (ECU) how much oxygen is found in the exhaust gases. This helps a vehicle run on a more efficient air/fuel ratio, allowing the engine to supply the converter with enough oxygen to complete the oxidation process.
Types of Catalytic Converters
As mentioned before, there are two primary catalysts – reduction and oxidation – that can be used within an exhaust system to handle specific gases.
Depending on the year of the vehicle and the type of catalytic converter it has, there might not be a reduction catalyst in place. There are two primary kinds of catalytic converters:
- Two-way: The two-way catalytic converter was present on vehicles in the United States until 1981. They only have oxidation catalysts, which help change carbon monoxide to carbon dioxide. Hydrocarbons (which is unburned and partially burned fuel) are changed to carbon dioxide and water.
- Three-way: Since 1981, the three-way catalytic converter has been used. This performs the same as the two-way converter with the addition of a reduction catalyst. As stated earlier, this is used to change nitrogen oxides to nitrogen and oxygen gases.
Diesel engines employ the use of two-way catalysts, and the converters are also specifically designed to work with diesel exhausts. The converters for these types of engines try and target particulates known as soluble organic fractions. These are made from hydrocarbons bound to soot.
Who Invented the Catalytic Converter?
The history of the catalytic converter dates to the end of the 19th century, when some prototypes were developed in France. In the mid-1950s Eugene Houdry, a French mechanical engineer, received a patent for his research to develop catalytic converters for gasoline engines.
Houdry’s development of the catalytic converter came from his concerns about the toll smokestack and automobile exhaust was having on air pollution. He had seen results of studies in Los Angeles and started working on converters for smokestacks.
Catalytic converters were further developed after the emissions control regulations that began in the early 1960s. The first production catalytic converter was created in 1973 at Engelhard Corporation, and widespread use of the part began around 1975.
How to Prevent Catalytic Converter Theft
Catalytic converters are often the target of thieves because the part contains valuable precious metals. Catalytic converter thefts often happen on vehicles with more ground clearance, since it’s easier to access the part.
Regardless of the type of vehicle you have, there are some steps you can take to help prevent theft:
- Park in well-lighted areas close to building entrances if a secure garage is unavailable.
- Weld the catalytic converter to the vehicle frame, which can make it harder to remove.
- Consider buying an aftermarket part similar to a metal cage that can be installed to cover the converter.
- Install a car alarm with a vibration alert sensor.
- Engrave your vehicle identification number (VIN) to the converter, which can help make selling the part harder and help alert you if your converter is stolen.
Signs of Catalytic Converter Issues
So what happens when a catalytic converter goes bad? Considering the role the part plays in a vehicle’s exhaust system, a range of symptoms can arise when it starts to experience wear and tear.
Some examples to watch out for include:
- Declining fuel efficiency: If a catalytic converter becomes clogged, it can reduce the amount of airflow through your engine. To compensate, your engine might start to burn more fuel than usual, resulting in a noticeable drop in fuel efficiency.
- Check warning light: A check engine light can indicate a range of things. However, there is a diagnostic system on cars manufactured after 1996 that will test the catalytic converter. If your converter is malfunctioning, the air-to-fuel ratio sensors might trigger the warning light to come on.
- Smelling rotten eggs: The catalytic converter might experience internal damage that causes it to have a hard time converting exhaust gases. The result can be a sulfuric “rotten egg” smell.
- Issues starting the engine: The exhaust gases in your vehicle have to escape. A clogged catalytic converter can prevent this from happening as effectively. This can result in increased exhaust pressure and cause your car to sputter or stall when you’re trying to get it going.
- Poor acceleration: Again, the exhaust gases have to escape somehow. Trapped exhaust and increased pressure from a clogged converter might cause you to have trouble accelerating your car. You might notice jerking or stalling when you try to do so.
- Failed emissions test: Many states require regular emissions testing on vehicles, and if you don’t pass yours the culprit very well could be your catalytic converter. Failing this test might be coupled with the other symptoms mentioned above.
Frequently Asked Questions
Why do people steal catalytic converters?
The number of catalytic converters reported as stolen has increased in 2022, partly due to the rising prices of different precious metals. The converter typically contains platinum, palladium and rhodium that can be sold to metal dealers.
What's in catalytic converters?
Typically, there are 3 to 7 grams of platinum in a standard catalytic converter. A standard converter also contains 2 to 7 grams of palladium and 1 to 2 grams of rhodium.

How much is a catalytic converter worth?
A recycler will pay between $50 and $250 for a catalytic converter, with some going for $800 to $1,500 if they come from hybrid vehicles.
Keep in mind that if your catalytic converter gets stolen, it could cost around $2,000 to replace it — all the more reason to take steps to prevent it from being stolen!
CATALYTIC CONVERTER BASICS

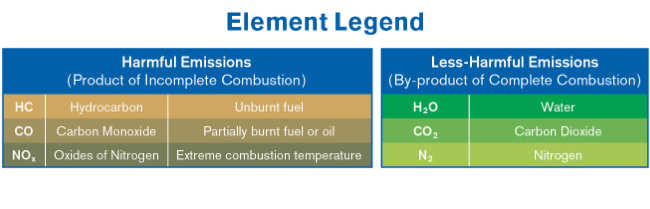
The catalytic converter is designed to convert harmful emissions produced by an internal combustion engine to less-harmful elements: H2O (water), CO2 (carbon dioxide) and N2 (nitrogen).
To perform this conversion, the catalytic converter works with a vehicle's PCM (Powertrain Control Module) and other emissions control devices. The OBDII (On-Board Diagnostics Version 2) system monitors the emissions control devices and provides feedback on their operating conditions. As the EPA (Environmental Protection Agency) updates emissions standards, the OBDII systems for newer vehicle models are calibrated by engineers to be more sensitive to fluctuations in emissions performance.
Catalytic converters can only function efficiently if the engine is operating properly and the exhaust system is leak-free. Malfunctioning sensors or actuators, damaged engine components, too-rich or too-lean fuel mixtures, and inconsistencies between engine cylinders may lead to converter efficiency issues and possibly damage the catalyst.
ANATOMY OF A CATALYTIC CONVERTER
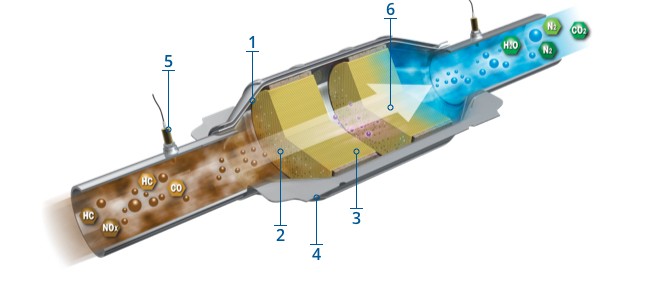
1 - Stainless Steel Body
Engineered for long life and durability. The ribbed body minimizes expansion and distortion, as well as forming pockets that protect the cushioning mat from direct exposure to exhaust gases.
2 - Monolithic Free-Flowing Substrate
The substrates are the backbone of the converter. This is where the proprietary mix of precious metals and the washcoat allow the conversion process to take place. Coatings are engineered for high temperature durability and optimal catalytic activity, as well as OBD system compatibility. Converters are available in single- or multiple-substrate designs.
3 - Catalyst Cushioning Mat
The mat cushions the converter substrate, holding the ceramic catalyst in proper alignment and accommodating for thermal expansion of the body.
4 - Head Shields to Match OE
Protects the vehicle’s undercarriage from heat created by the chemical reactions in the converter.
5 - O2 (Oxygen) Sensor Ports
Another vital part of an emissions control system, these sensors are placed before and after the catalytic converter on an OBDII vehicle. They are designed to monitor the O2 levels in the exhaust gas and are also used to evaluate the status of the converter. This information along with data provided by the mass air flow, MAP (manifold absolute pressure) and engine sensors, allow the PCM to adjust fuel controls.
6 - Precious Metals/Catalysts
The precious metals introduced onto the substrate serve as catalysts that chemically process raw exhaust gases. Those most commonly used are platinum (Pt), palladium (Pd) and rhodium (Rh).


Leave a comment